By Christina McAninch
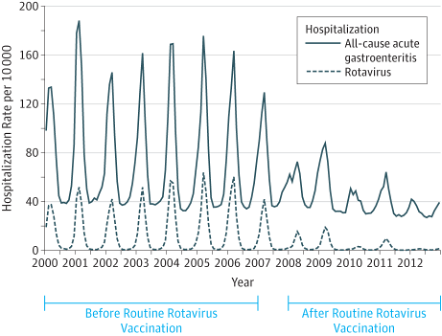
Rotavirus is a highly contagious virus that causes gastroenteritis, or inflammation of the stomach and intestines. It is a leading cause of severe diarrhea and dehydration in infants and young children. Before there was a vaccine (Figure 1), almost all children would get infected before age five. According to the Centers for Disease Control (CDC), there would be over 400,000 doctor visits, over 200,000 ER visits, 55,000-70,000 hospitalizations, and 20-60 deaths every year in the United States. Globally, there were up to 500,000 deaths per year, mostly in developing countries, due to limited access to medical care, poor hygiene, and malnutrition. In 2006, a safe and effective vaccine, RotaTeq, was released. In 2008, a second vaccine, Rotarix, was approved. In the United States, from 2008-2012, rotavirus-related hospitalizations declined by 63-94% each year (Figure 2).1 Globally, from 2006-2019, the vaccine saved an estimated 140,000 lives per year.2
While the rotavirus vaccine has drastically reduced disease burden, only 70-75% of children in the U.S. receive the full series, which is a lower uptake than other childhood vaccines.3 This percentage is even lower for children who are uninsured (56.5%) or below the federal poverty level (66.8%).4 Suboptimal vaccine uptake not only results in preventable illnesses, hospitalizations, and ER visits, but it also prevents the establishment of herd immunity. Herd immunity occurs when enough people in a community, through vaccination or natural infection, are immune to a disease so that it no longer spreads easily.5, 6 Importantly, obtaining herd immunity protects those who cannot be vaccinated, such as cancer patients and those with immune disorders. Therefore, reduced uptake of the rotavirus vaccine can cause harm to vulnerable individuals.
Vaccination mimics natural infection without the negative consequences of natural infection.7, 8 One response to receiving vaccines is the production of tumor necrosis factor-alpha (TNF-α). TNF-α is a chemical messenger, known as a cytokine, that promotes inflammation and is produced primarily by immune cells.9 It is crucial in helping the immune system fight off pathogens, such as disease-causing bacteria and viruses.10-12 However, overproduction of TNF-α can be damaging and is implicated in diseases such as Crohn’s disease, or CD.13
CD is one of the major forms of inflammatory bowel disease.14 It is a chronic, debilitating disease in which the immune system overreacts to bacteria in the gastrointestinal tract, resulting in chronic inflammation.15 Symptoms include abdominal pain, diarrhea, weight loss, and fatigue.16 Tumor necrosis factor (TNF) blockers are a first-line treatment for moderate to severe Crohn’s disease and include Humira, Remicade, Simponi, and Cimzia.17 These medications bind to TNF-α, neutralizing its activity and reducing inflammation. For CD patients, neutralizing TNF-α can treat symptoms by suppressing chronic inflammation, promoting healing, and preventing disease.18-20
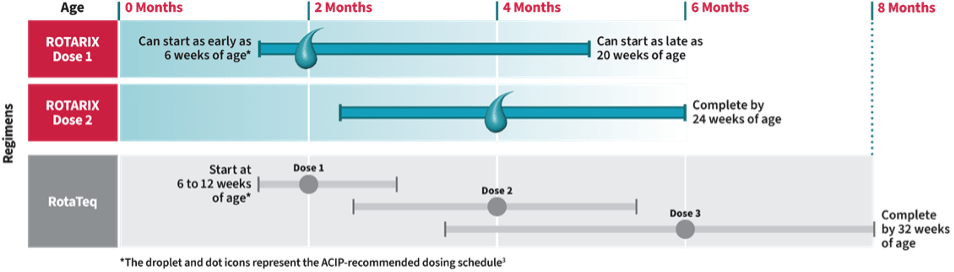
Patients with CD can have a healthy pregnancy with good planning and medical oversight. They are advised to conceive while in remission and to continue their medications throughout pregnancy to maintain remission and prevent complications.21-23 Most IBD medications, including TNF blockers, are considered safe during pregnancy.24 However, these drugs are, by design, immunosuppressive and can interfere with the immune response to vaccines.7 Since these medications can remain in a baby’s system for up to one year after birth, there has been concern regarding the rotavirus vaccine, the only live-attenuated vaccine routinely given in the first year of life in the United States (Figure 3).25
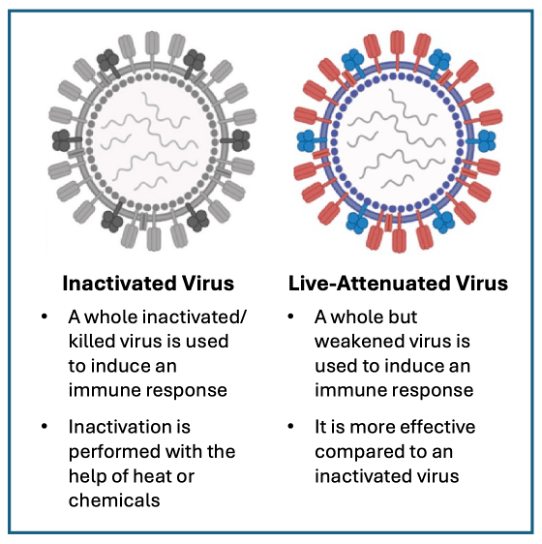
There are several different types of vaccines, each of which is designed to generate an immune response and teach the immune system how to fight the virus or bacteria in the future (Figure 3). Live-attenuated vaccines (LAVs) contain weakened versions of live virus or bacteria to mimic natural infection and generate an immune response without causing serious illness.26 For example, Rotarix was made by growing the human strain of rotavirus in non-human cells, causing it to weaken so that it wouldn’t cause disease but could still generate an immune response.27, 28 RotaTeq was made using a calf rotavirus and swapping genes with the human virus to eliminate the disease-causing genes while retaining the genes that promote immunity.29 The rotavirus, MMR, and chicken pox vaccines are LAVs recommended in the U.S. Child and Adolescent Immunization Schedule. The adenovirus, typhoid, and bacillus Calmette-Guérin (tuberculosis) vaccines are LAVs that are not routinely recommended in the U.S. except for certain high-risk groups, such as travelers, military, or health care workers. Compared to vaccines with inactivated virus, LAVs generate a robust immune response and often provide life-long protection.26 However, while highly effective, some LAVs carry a small, but real risk of infection due to uncontrolled viral replication, especially in immunocompromised individuals.6
Out of an abundance of caution, it has previously been recommended that infants exposed to TNF blockers in utero delay live-attenuated vaccines until the medications are undetectable or 6-12 months of age, which is past the recommended age for the rotavirus vaccine (Figure 4).23 This concern is well-founded—a healthy three-month-old infant whose mother had been treated with Remicade for Crohn’s disease died of tuberculosis after receiving the bacillus Calmette-Guérin vaccine.30 However, unlike the BCG vaccine, the risk associated with the rotavirus vaccine is merely theoretical, warranting further investigation.25
A 2025 review in the journal Inflammatory Bowel Diseases assessed the safety of the rotavirus vaccine in infants exposed to IBD medications in utero.25 The authors identified ten studies involving 300 infants who had received rotavirus vaccination.31-40 These infants had been exposed to TNF blockers or biologics targeting other inflammation-promoting proteins (i.e. Entyvio, Stelara, Skyrizi, and Omvoh). In seven of the studies, no adverse events of any kind were found to be associated with the vaccine.33-35, 37-40 In a study of 133 infants, three required medical attention for reasons unrelated to the vaccine.36 One study compared fourteen exposed infants with seventy-three non-exposed infants and found no significant difference in adverse events between the two groups.39 In a study of thirty-nine infants, six infants experienced fever and one diarrhea, temporary side effects, at rates comparable to the general population.31 Based on these findings, the authors concluded that infants exposed to TNF blockers and other IBD medications in utero can safely receive the rotavirus vaccine.
Vaccines are among the most effective medical interventions in history, having saved an estimated 154 million lives, mostly children, from 1974 to 2024, according to a study in The Lancet.41 Some people cannot be vaccinated due to legitimate medical concerns. It is important to investigate these concerns and to educate the public so that as many children as possible can be vaccinated and live long and healthy lives.
TL; DR:
- Rotavirus is a highly contagious virus and can be life-threatening.
- Released in 2006, the rotavirus vaccine is safe and effective.
- Evidence shows that the rotavirus vaccine is safe for babies exposed to TNF blockers, such as treatments for Crohn’s disease, in utero.
Reference
- Leshem E, Tate JE, Steiner CA, et al. Acute gastroenteritis hospitalizations among US children following implementation of the rotavirus vaccine. JAMA 2015;313:2282-4.
- Clark A, Mahmud S, Debellut F, et al. Estimating the global impact of rotavirus vaccines on child mortality. Int J Infect Dis 2023;137:90-97.
- Ai CE, Steele M, Lopman B. Disease burden and seasonal impact of improving rotavirus vaccine coverage in the United States: A modeling study. PLoS One 2020;15:e0228942.
- Pindyck T, Tate JE, Parashar UD. A decade of experience with rotavirus vaccination in the United States – vaccine uptake, effectiveness, and impact. Expert Rev Vaccines 2018;17:593-606.
- Desai AN, Majumder MS. What Is Herd Immunity? JAMA 2020;324:2113.
- Pollard AJ, Bijker EM. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol 2021;21:83-100.
- Visser LG. TNF-alpha Antagonists and Immunization. Curr Infect Dis Rep 2011;13:243-7.
- Otten AT, Bourgonje AR, Horinga PP, et al. Use of Tumor Necrosis Factor-alpha Antagonists Is Associated With Attenuated IgG Antibody Response Against SARS-CoV-2 in Vaccinated Patients With Inflammatory Bowel Disease. Front Immunol 2022;13:920333.
- Skartsis N, Ferreira LMR, Tang Q. The dichotomous outcomes of TNFalpha signaling in CD4(+) T cells. Front Immunol 2022;13:1042622.
- Strangfeld A, Listing J. Infection and musculoskeletal conditions: Bacterial and opportunistic infections during anti-TNF therapy. Best Pract Res Clin Rheumatol 2006;20:1181-95.
- Mehta AK, Gracias DT, Croft M. TNF activity and T cells. Cytokine 2018;101:14-18.
- Ruby J, Bluethmann H, Peschon JJ. Antiviral activity of tumor necrosis factor (TNF) is mediated via p55 and p75 TNF receptors. J Exp Med 1997;186:1591-6.
- Souza RF, Caetano MAF, Magalhaes HIR, et al. Study of tumor necrosis factor receptor in the inflammatory bowel disease. World J Gastroenterol 2023;29:2733-2746.
- Scott FI, Osterman MT. Medical management of crohn disease. Clin Colon Rectal Surg 2013;26:67-74.
- Haag LM, Siegmund B. Intestinal Microbiota and the Innate Immune System – A Crosstalk in Crohn’s Disease Pathogenesis. Front Immunol 2015;6:489.
- Veauthier B, Hornecker JR. Crohn’s Disease: Diagnosis and Management. Am Fam Physician 2018;98:661-669.
- Purnak T, Ertan A. Optimal Management of Patients with Moderate-to-Severe Inflammatory Bowel Disease. J Clin Med 2024;13.
- Adegbola SO, Sahnan K, Warusavitarne J, et al. Anti-TNF Therapy in Crohn’s Disease. Int J Mol Sci 2018;19.
- Chowers Y, Allez M. Efficacy of anti-TNF in Crohn’s disease: how does it work? Curr Drug Targets 2010;11:138-42.
- Billmeier U, Dieterich W, Neurath MF, et al. Molecular mechanism of action of anti-tumor necrosis factor antibodies in inflammatory bowel diseases. World J Gastroenterol 2016;22:9300-9313.
- Hashash JG, Kane S. Pregnancy and Inflammatory Bowel Disease. Gastroenterol Hepatol (N Y) 2015;11:96-102.
- Baiocco PJ, Korelitz BI. The influence of inflammatory bowel disease and its treatment on pregnancy and fetal outcome. J Clin Gastroenterol 1984;6:211-6.
- van der Woude CJ, Ardizzone S, Bengtson MB, et al. The second European evidenced-based consensus on reproduction and pregnancy in inflammatory bowel disease. J Crohns Colitis 2015;9:107-24.
- Nielsen OH, Loftus EV, Jr., Jess T. Safety of TNF-alpha inhibitors during IBD pregnancy: a systematic review. BMC Med 2013;11:174.
- Schell TL, Fass L, Hitchcock ME, et al. Safety of Rotavirus Vaccination in Infants That Were Exposed to Biologics In Utero: A Systematic Review. Inflamm Bowel Dis 2025;31:1789-1796.
- Tang YD, Li Y, Cai XH, et al. Viral Live-Attenuated Vaccines (LAVs): Past and Future Directions. Adv Sci (Weinh) 2025;12:e2407241.
- Fukuda S, Kugita M, Kumamoto K, et al. Generation of Recombinant Authentic Live Attenuated Human Rotavirus Vaccine Strain RIX4414 (Rotarix((R))) from Cloned cDNAs Using Reverse Genetics. Viruses 2024;16.
- Bernstein DI, Smith VE, Sherwood JR, et al. Safety and immunogenicity of live, attenuated human rotavirus vaccine 89-12. Vaccine 1998;16:381-7.
- Heaton PM, Goveia MG, Miller JM, et al. Development of a pentavalent rotavirus vaccine against prevalent serotypes of rotavirus gastroenteritis. J Infect Dis 2005;192 Suppl 1:S17-21.
- Cheent K, Nolan J, Shariq S, et al. Case Report: Fatal case of disseminated BCG infection in an infant born to a mother taking infliximab for Crohn’s disease. J Crohns Colitis 2010;4:603-5.
- Beaulieu DB, Ananthakrishnan AN, Martin C, et al. Use of Biologic Therapy by Pregnant Women With Inflammatory Bowel Disease Does Not Affect Infant Response to Vaccines. Clin Gastroenterol Hepatol 2018;16:99-105.
- Chiarella-Redfern H, Lee S, Jubran B, et al. Suboptimal Vaccination Administration in Mothers With Inflammatory Bowel Disease and Their Biologic-Exposed Infants. Inflamm Bowel Dis 2022;28:79-86.
- Prieto-Pena D, Calderon-Goercke M, Adan A, et al. Efficacy and safety of certolizumab pegol in pregnant women with uveitis. Recommendations on the management with immunosuppressive and biologic therapies in uveitis during pregnancy. Clin Exp Rheumatol 2021;39:105-114.
- Weiss B, Ben-Horin S, Lev A, et al. Immune function in newborns with in-utero exposure to anti-TNFalpha therapy. Front Pediatr 2022;10:935034.
- Esteve-Sole A, Deya-Martinez A, Teixido I, et al. Immunological Changes in Blood of Newborns Exposed to Anti-TNF-alpha during Pregnancy. Front Immunol 2017;8:1123.
- Fitzpatrick T, Alsager K, Sadarangani M, et al. Immunological effects and safety of live rotavirus vaccination after antenatal exposure to immunomodulatory biologic agents: a prospective cohort study from the Canadian Immunization Research Network. Lancet Child Adolesc Health 2023;7:648-656.
- Lee KE, Jung SA, Park SH, et al. Influence of anti-tumor necrosis factor-alpha therapy to pregnant inflammatory bowel disease women and their children’s immunity. Intest Res 2019;17:237-243.
- Ernest-Suarez K, Murguia-Favela LE, Novak KL, et al. Normal Infant Immunologic Assessment and Uneventful Live Rotavirus Vaccination Despite Continuous Tofacitinib Exposure In Utero and During Breastfeeding. Crohns Colitis 360 2024;6:otae006.
- Moens A, van der Woude CJ, Julsgaard M, et al. Pregnancy outcomes in inflammatory bowel disease patients treated with vedolizumab, anti-TNF or conventional therapy: results of the European CONCEIVE study. Aliment Pharmacol Ther 2020;51:129-138.
- Mitrova K, Pipek B, Bortlik M, et al. Safety of Ustekinumab and Vedolizumab During Pregnancy-Pregnancy, Neonatal, and Infant Outcome: A Prospective Multicentre Study. J Crohns Colitis 2022;16:1808-1815.
- Shattock AJ, Johnson HC, Sim SY, et al. Contribution of vaccination to improved survival and health: modelling 50 years of the Expanded Programme on Immunization. Lancet 2024;403:2307-2316.