By: Abbey Rebok
For over half a century, fluoride has been supplemented to drinking water to prevent tooth decay. While initially deemed as a major public health advancement, skepticism has been brewing over the necessity and safety of fluoridation. Critics of fluoridation suggest fluoride imparts an unnecessary risk to public health, while proponents boast of its notable success in fighting tooth decay. These conflicting views make it difficult to decipher reality versus fallacy, so what does the science say? Is fluoride safe?
What is fluoridation?
The fluoridation process involves adding a fluoride-containing compound to water. The three most used compounds in the United States are sodium fluoride, sodium fluorosilicate, and fluorosilicate acid. While fluoride compounds are added to dental products, fluoride is also naturally present in water due to its release from rocks and soil. The process of fluoridation began in some parts of the United States in 1945 when scientists correlated higher levels of fluoride in drinking water to decreased occurrence of cavities1. As will be discussed in greater detail below, a variety of government agencies regulate fluoride in drinking water. The U.S. Public Health Service (PHS) forms recommendations for optimal fluoride concentrations in public water systems, while the U.S. Environmental Protection Agency (EPA) regulates the maximum allowable amount of fluoride in such systems. Additionally, since bottled water falls under the guidance of the U.S. Food and Drug Administration (FDA), the FDA enforces its own regulations on optimal fluoride levels in bottled beverages. As a result, the recommended and maximum allowable amounts of fluoride can vary by source.
In 1962, the PHS recommended adding fluoride to public water systems at concentrations between 0.7 to 1.2 milligrams fluoride to 1 liter of water (mg/L). However, the recommended fluoride concentration was lowered to 0.7 mg/L in 2015 to account for the increased prevalence of fluoride in other sources, such as dental products. Beyond public water systems, fluoride is also routinely found in bottled water via both natural and artificial means. The FDA limits added fluoride in bottled water to 0.7 mg/L but permits levels up to 2.4 mg/L if the fluoride is naturally present. On average, fluoride is naturally present in water sources at 0.2 mg/L,but levels can vary significantly by region. (Figure 1).
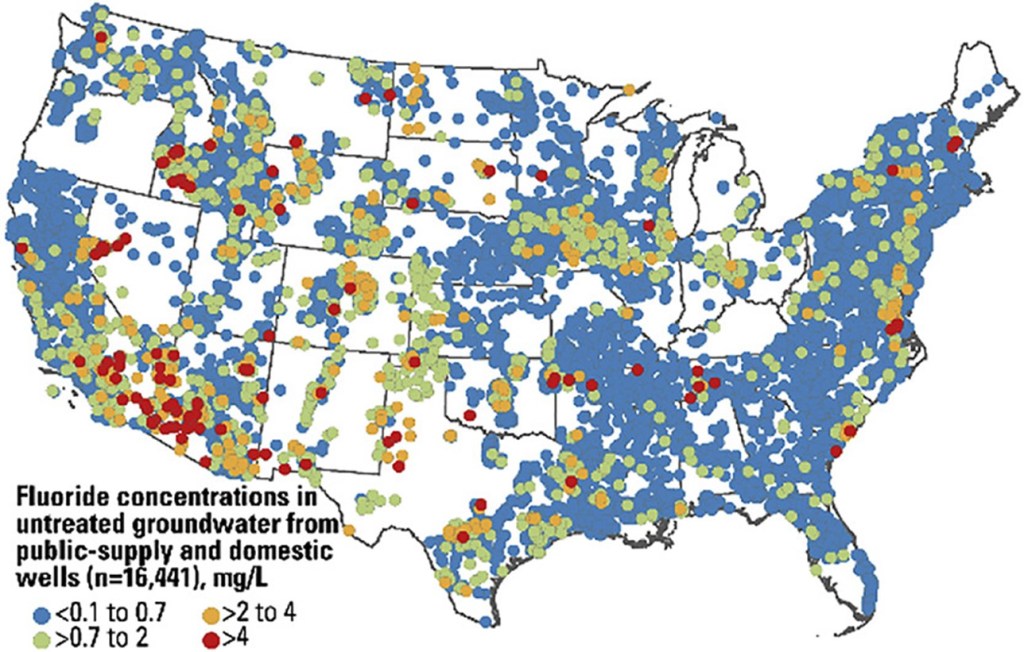
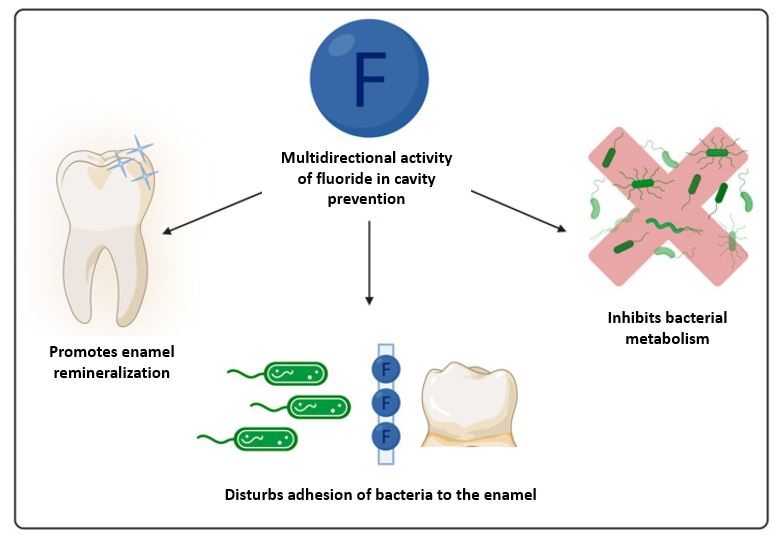
Fluoride prevents tooth decay
The fluoridation of drinking water aims to prevent tooth decay and cavities. Tooth decay occurs when bacteria consume sugar and produce acid, ultimately leading to the breakdown of teeth over time. Fluoride combats tooth decay in a dual approach by acting upon teeth and the bacteria themselves (Figure 2). Fluoride tends to accumulate in areas high in calcium, such as teeth and bones, and helps remineralize the enamel, thus strengthening teeth and potentially reversing early tooth decay. Fluoride disrupts essential metabolic processes, ultimately leading to bacterial death, by binding and inhibiting certain bacterial enzymes (proteins that accelerate the speed of reactions). Moreover, weak acids, such as hydrogen fluoride, acidify bacterial cells and inhibit pH sensitive metabolic processes3.
It is estimated that for every $1 spent on fluoridation, $38 is saved on dental bills. Fluoridated water alone is estimated to prevent 25% of cavities and is deemed one of the greatest public health achievements of the 20th century. Indeed, fluoridation has greatly improved oral health, but are there any drawbacks?
Moderation is key: excessive fluoride may pose health risks
Fluoridating public water systems and bottled water to meet recommended levels is not mandatory, but the EPA has set a strict maximum level of fluoride to 4 mg/L. As with most good things in life, too much fluoride poses multiple health risks, as chronic exposure to high levels of fluoride is detrimental to one’s health5. Fluoride that is not excreted from the body accumulates in calcium-rich areas, such as in bones and teeth. Low levels of fluoride are largely considered to be safe; however, too much fluoride can weaken bones and lead to skeletal fluorosis.
Skeletal fluorosis may include hyperostosis (abnormal bone growth) and osteoporosis (decreased bone density). While not common in the United States, skeletal fluorosis is endemic in India and China due to excess fluoride routinely present in groundwater. In India, the average fluoride concentration in drinking water is estimated to be 2.37 mg/L, but some studies have reported fluoride levels upwards of 9.22 mg/L.
While less detrimental than skeletal fluorosis, elevated fluoride levels can also lead to dental fluorosis, which affects approximately 23% of the population in the United States. Exposure to excessive fluoride during tooth enamel formation in children can prevent its proper formation and lead to discoloration and, in rare cases, may cause tooth damage. However, the vast majority of dental fluorosis cases are mild and do not compromise oral health. To mitigate dental fluorosis, the EPA suggests children are not exposed to more than 2 mg/L fluoride in drinking water. Since this is below the maximum level allowed of 4 mg/L fluoride, it is not an enforceable regulation. However, the public must be notified if their publicly available water contains fluoride at concentrations greater than 2.0 mg/L.
Does fluoride cause cancer?
A major public health concern regarding high level fluoride exposure is cancer, specifically osteosarcoma (bone cancer). The theory that fluoride may promote formation of osteosarcoma is not far-fetched, since fluoride tends to accumulate in bones actively growing (termed growth plates, which are common sites for bone cancers), and fluoride at high levels is known to promote abnormal bone growth. In 1990, the U.S. National Toxicology Program (NTP) published findings that indicated male rats developed osteosarcoma following chronic administration of sodium fluoride. Only 4 out of 130 male rats developed osteosarcoma after exposure to high levels (25-175 mg/L) of sodium fluoride for 2 years. Importantly, the other experimental groups (female rats and both male and female mice) did not develop osteosarcoma6. The lack of data reproducibility may suggest the results are gender and species dependent. In fact, there is no consistent evidence to suggest high fluoride levels promote cancers, such as osteosarcoma, in humans. In response to the NTP study, the PHS released a report in 1991, comprising more than 50 epidemiological studies spanning over 40 years, that concluded the recommended levels of fluoride in drinking water did not pose a significant cancer risk. Additionally, analyses of international public health data report that individuals in regions with higher fluoride levels do not have a significant increase of cancer incidence7.
Do recommended levels of fluoride impact neurodevelopment?
In 2016, the NTP began to investigate the role of fluoride in neurodevelopment and cognition. By analyzing published epidemiology studies in non-U.S. countries, such as Canada, China, India, among others, it was identified with moderate confidence, meaning the results of 18 out of 19 human studies determined to have a low bias potential were concordant, that fluoride concentrations greater than 1.5 mg/L are associated with decreased IQ scores8.
The implications of these results are already mobilizing efforts to address the findings. In the September 2024 court hearing of Food & Water Watch, Inc., et al. v. EPA, California Federal District Court Judge Edward Chen ruled that the presence of fluoride in drinking water presented a potentially significant public health risk, citing preliminary results from the NTP investigation, and ordered the EPA to review fluoride regulations. It’s important to note the NTP findings indicated a risk with fluoride concentrations greater than 1.5 mg/L. Current recommended fluoride levels are 0.7 mg/L, and while there are no studies indicating whether these concentrations pose a public health risk, additional research should be conducted to clarify the matter.
Takeaways
It is evident that high levels of fluoride pose a health risk, and efforts should be made to stay within federal recommendations. With that being said, the maximum allowable amount of fluoride in public water systems is 4 mg/L, which is much higher than concentrations suggested to decrease IQ scores. Fluoride levels less than 0.7 mg/L have not been shown to cause adverse health effects. Moving into 2025, it will be vital for the EPA to address the concerns raised by the NTP and determine if the findings warrant revising regulations to reduce the maximum allowable amount of fluoride in drinking water.
TL;DR
- Fluoride is naturally found in water sources but can also be artificially added to prevent tooth decay and cavities.
- Chronic exposure to high levels of fluoride may dysregulate normal bone function.
- Limited evidence suggests elevated fluoride levels may be associated with lowered IQ scores in children and development of cancer.
Reference
- Dean, H. T., Francis A. Arnold, Jr., & Elias Elvove. (1942). Domestic Water and Dental Caries: V. Additional Studies of the Relation of Fluoride Domestic Waters to Dental Caries Experience in 4,425 White Children, Aged 12 to 14 Years, of 13 Cities in 4 States. Public Health Reports (1896-1970), 57(32), 1155–1179. https://doi.org/10.2307/4584182
- McMahon, P. B., Brown, C. J., Johnson, T. D., Belitz, K., & Lindsey, B. D. (2020). Fluoride occurrence in United States groundwater. The Science of the total environment, 732, 139217. https://doi.org/10.1016/j.scitotenv.2020.139217
- Marquis, R. E., Clock, S. A., & Mota-Meira, M. (2003). Fluoride and organic weak acids as modulators of microbial physiology. FEMS microbiology reviews, 26(5), 493–510. https://doi.org/10.1111/j.1574-6976.2003.tb00627.x
- Piszko, A., Piszko, P. J., Lubojański, A., Grzebieluch, W., Szymonowicz, M., & Dobrzyński, M. (2023). Brief Narrative Review on Commercial Dental Sealants-Comparison with Respect to Their Composition and Potential Modifications. Materials (Basel, Switzerland), 16(19), 6453. https://doi.org/10.3390/ma16196453
- Solanki, Y. S., Agarwal, M., Gupta, A. B., Gupta, S., & Shukla, P. (2022). Fluoride occurrences, health problems, detection, and remediation methods for drinking water: A comprehensive review. The Science of the total environment, 807(Pt 1), 150601. https://doi.org/10.1016/j.scitotenv.2021.150601
- National Toxicology Program (1990). NTP Toxicology and Carcinogenesis Studies of Sodium Fluoride (CAS No. 7681-49-4) in F344/N Rats and B6C3F1 Mice (Drinking Water Studies). National Toxicology Program technical report series, 393, 1–448.
- Overall evaluations of carcinogenicity: an updating of IARC Monographs volumes 1 to 42. (1987). IARC monographs on the evaluation of carcinogenic risks to humans. Supplement, 7, 1–440.
- Taylor, K. W., Eftim, S. E., Sibrizzi, C. A., Blain, R. B., Magnuson, K., Hartman, P. A., Rooney, A. A., & Bucher, J. R. (2025). Fluoride Exposure and Children’s IQ Scores: A Systematic Review and Meta-Analysis. JAMA pediatrics, 10.1001/jamapediatrics.2024.5542. Advance online publication. https://doi.org/10.1001/jamapediatrics.2024.5542